FDA Okays Senza Spinal Cord Stimulation System for Pain
DRUG & REFERENCE INFORMATION
Nevro Corp has received an "approvable" letter from the US Food and Drug Administration (FDA) for its spinal cord stimulation (SCS) system for treatment of chronic pain.
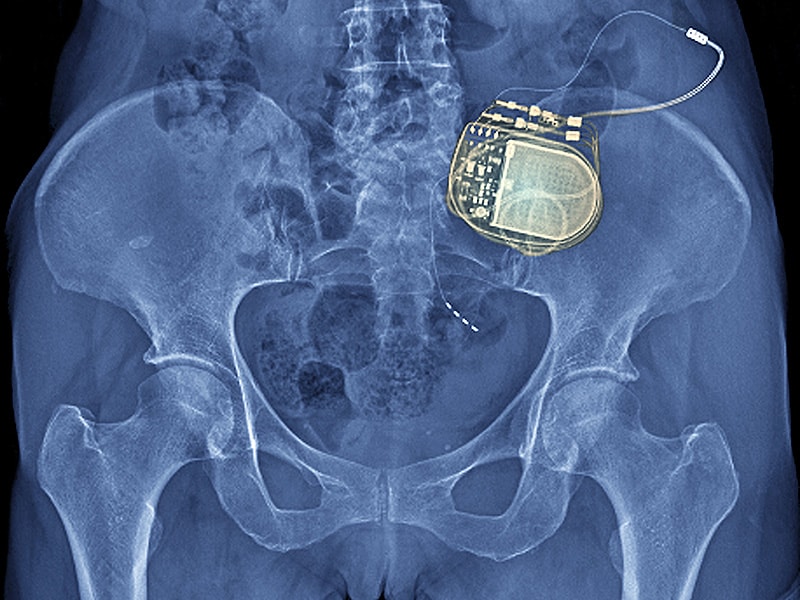
The Senza SCS system delivers Nevro's proprietary HF10therapy, "an advanced SCS therapy that provides electrical pulses to the spinal cord at a rate up to 10,000 per second (10 kHz), as compared to traditional SCS, which utilizes low frequency stimulation, typically between 40 Hz and 60 Hz," the company explains on its website.
Unlike traditional SCS therapy, HF10 therapy relieves back and leg pain without causing a constant tingling sensation. The electrical pulses are delivered by small electrodes on leads placed near the spinal cord and connected to a compact, battery-powered generator implanted under the skin. The system features remote control with patient-adjustable stimulation levels and uses a rechargeable battery that lasts 10 years under typical settings.
In a prospective observational study, 72 of 82 (88%) patients implanted with the system reported significant improvement in their chronic low back pain and opted to continue using it (Pain Med. 2014;15:347-354).
A randomized controlled trial of the Senza SCS system, completed in 2014, enrolled 241 patients and provided a long-term comparison of the HF10 therapy to traditional SCS for back and leg pain. The results were presented during a plenary session last month at the 2014 North American Neuromodulation Society (NANS) annual meeting.
Approval of the premarket approval application (PMA) for Senza is subject to satisfaction of regulatory inspections and audits of manufacturing facilities, methods, and controls for Senza to ensure compliance with the FDA's Quality System Regulation, as well as finalization of the product's labeling with the FDA.
"We are pleased that the FDA has determined our Senza SCS system to be approvable based on the strength of the data provided in the PMA," Michael DeMane, Nevro's chairman and chief executive officer, said in a statement. "We are working to satisfy the conditions of approval and anticipate initial commercial availability in the U.S. by mid-2015," he added.
The Senza system is commercially available in Europe and Australia, where over 2500 patients have been treated to date, the company says.

No comments:
Post a Comment